Hope Biosciences Annual Report - 2022
- Hope Biosciences
- Dec 22, 2022
- 3 min read
Updated: 5 days ago
Looking at some of the year end numbers, it’s safe to say we shocked even ourselves. Companies typically release their year-end results to the public as a sign of progress and success. But instead of posting a balance sheet, we wanted to share a different set of tallies. To get a real sense of the process, it is revelatory to note that it took over 4,000 media flasks, 2,500 bunny suits, and 17,000 agar plates for Hope Bio to culture over 116 BILLION stem cells for banking and clinical research.
All these numbers quantifying our accomplishments translate into something not so easily quantifiable: the amount of Hope created.

Mesenchymal Stem Cell therapeutics manufactured by Hope Biosciences are being used in over 35 clinical trial and expanded access protocols. Over 1,500 treatments have been safely administered, including intravenous, intra-articular, and intrathecal administrations. The published and peer-reviewed results of our cells against Rheumatoid Arthritis and Lupus are now available to the public. More clinical results are forthcoming from the TBI trial that concluded this year with UTHealth and the Longhaul Covid trials using our cells.

OUR CELLS IN ACTION: read the peer-reviewed & published clinical results now.
With all the fast-paced progress our team has made for the biotech and healthcare industries, our company has held onto our status as industry leaders. To our knowledge, we are the only organization able to manufacture consistent treatments designed for repeatability—a necessary component for cell therapeutics. If you have an in vitro, preclinical or clinical project that needs a cellular product, please propose your project online at www.hope.bio/research-with-hope. We are excited to collaborate with others in advancing the understanding and application of our cells.

Hope Biosciences's patented products are not only available for stem cell research, but also beauty and skincare innovation. We officially entered the skincare and beauty industries in January of 2022 with the release of Wondercell® Gel, a multi-use skin serum. Wondercell Gel’s active ingredient, Wonderstem®, is our patented conditioned media containing live growth factors derived from adult stem cells, and it has the beauty industry buzzing about biotech. After the release of Wondercell® Gel, we had the pleasure of collaborating with Droplette Inc. for the release of their Growth Factor Capsules, powered by Wonderstem®. Their award-winning, medical-turned-cosmetic skin infusion device is capable of delivering molecules like Growth Factors beyond the skin’s surface.

As the calendar year draws to a close, we continue to build on the progress we’ve made heading into 2023. In fact, we are kicking off the new year in style at the 41st Annual JP Morgan Health Care Conference in San Francisco beginning January 9th and will live tweet throughout the event, so follow us on Twitter now. There is tremendous work being done behind the scenes to lay the groundwork for upcoming expansions in operations and research. Stay tuned into our progress by subscribing to our monthly leadership address from our CEO and Founder, Donna Chang.
Our banking services for adult and newborn clients are slated to add many more people to our Hope family as we continue offering the peace of mind that comes with owning a Master Cell Bank at Hope. On a popular sentiment from 2022, we didn’t come this far to only come this far. See you in 2023!



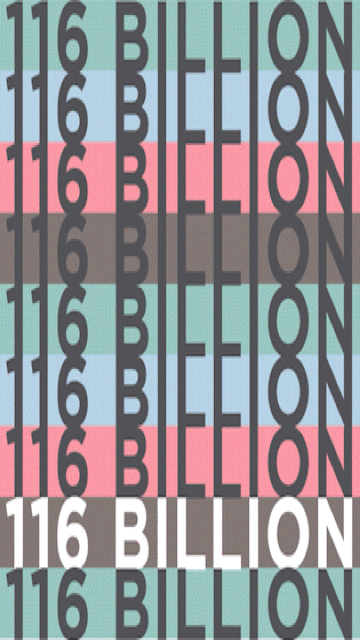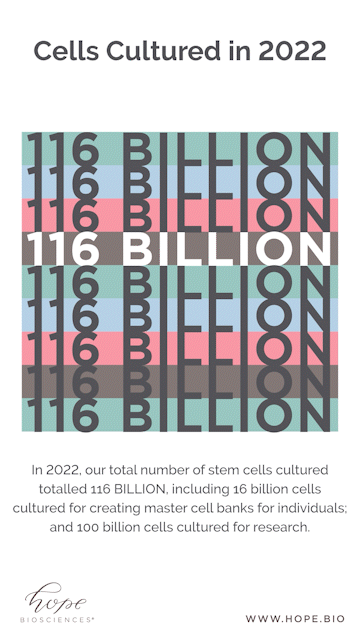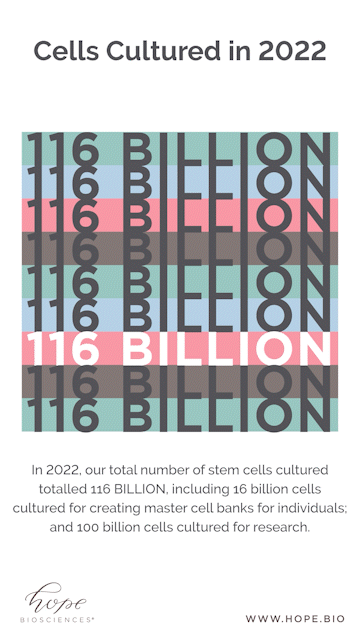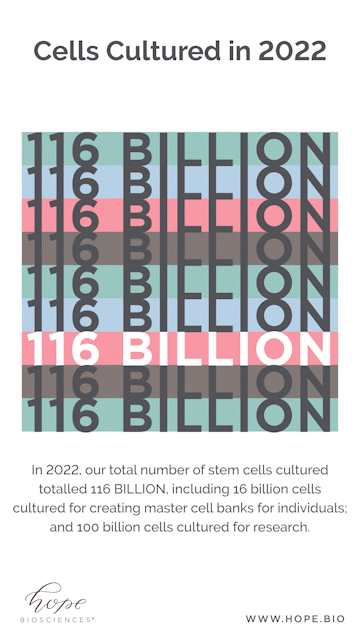










Comments